Manage ISO 14971 risks, CEI 62366 usability and the design of your medical devices with Medical Device Software
Software tools for medical devices
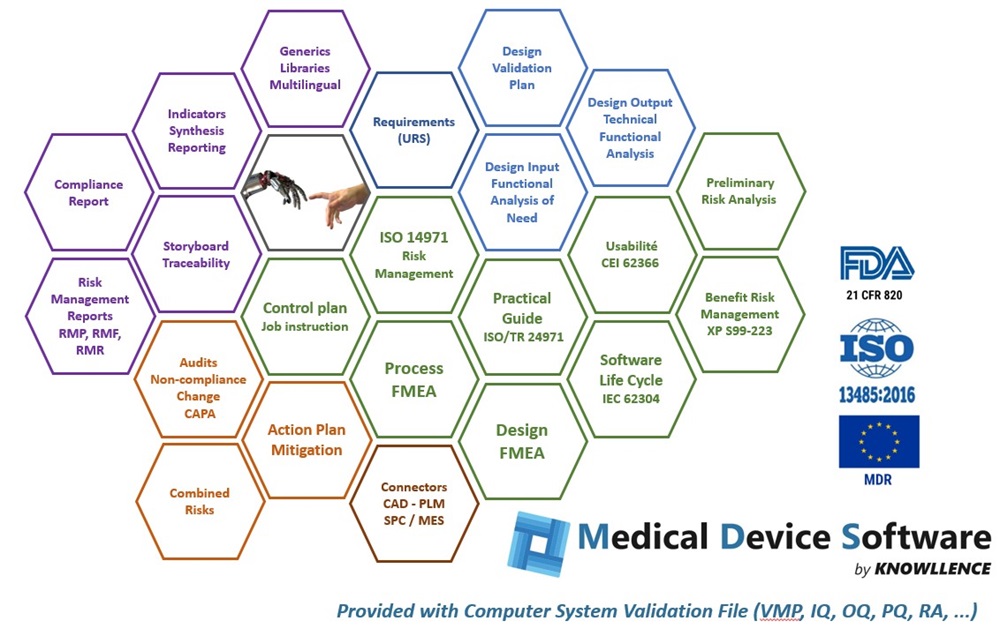
Medical Device Software is comprised of different data-sharing software modules that do away with duplicate entries and any risk of inconsistency:
- RM 14971: risk management following ISO 14971
- Need: to integrate your customers’ viewpoints and structure the “real” needs with functional analysis
- Structure: to cross-reference needs and technical nomenclature or to optimize the (re)design of medical devices
- FMEA : for Design and/or Process FMEAs, control plans, manufacturing flow charts, workstation charts, and so on.
The modular programs in Medical Device Software provide the following advantages to Regulatory Affairs and Design teams:
- Structures functional analysis, usability, FMEA, and risk analysis processes
- Secures audits (compliance, completeness, up to date, traceability, etc.)
- Ensures coherence of the CEI 62366 Usability Test Report and the ISO 14971 Risk Management Report
- Saves significant time on updates, management of the medical device versions, printing of reports, and so forth
- Manages the project complexities
- Motivates teams by getting them involved
Facilitate cooperation between Regulatory Affairs and Design
Medical Device Software is a software package that manages ISO 14971 risks, CEI 62366 usability, and the design of your medical devices.
It is a software solution for:
- Regulatory Affairs requirements in terms of ISO 14971 risk management
- Designers through functional analyses (needs and technical) and FMEAs
The goal: sharing information, pooling, and making the most of your usability, design, and risk data for your medical devices.
High-performance tools for medical device manufacturers!
With Medical Device Software, you’ll have a permanent solution to:
- Carry out ISO 14971 risk management analyses within the framework of regulatory affairs
- Build the CEI 62366 Usability Test Report coherently
- Perform functional analyses and Design FMEA and Process FMEA studies
- Guarantee the overall coherence of this data over time
Depending on your context and your needs, you can use either one module, or broaden to the sharing, consistency, and automation between the various modules in this single database.
The biggest advantage of Medical Device Software is quick start-up
- We can take care of the switch over from your current IT tools if you prefer. Confide this tedious task to us and retrieve your existing studies!
- Our expertise in computerizing standards, best practices, and reference documents provides Medical Device Software with a ready-to-use configuration. Since our product is the most customizable one on the market, we can adapt it to your internal risk management standards as well.
- You can take advantage of the pre-integrated content libraries by modifying and enhancing them to include dangers, dangerous occurrences, and harm to humans, equipment, or the environment, and so on. Your specific references can be filled in and used as needed: dangerous situations, actions to perform, etc.
- Begin with the software module(s) adapted to your current situation.
Save time and be more efficient in the long run
- For each new medical device project, reuse your libraries or any generic studies you have and take advantage of the possibility to manage different versions of medical devices.
- Post-market data is easily identified through our unique, automatic data-tracking system. With just one click, you’ll know who did what, when, and how.
- Your projects can be built in the various languages you use for your business—for the interface and documents, but especially for each datum.
- Medical Device Software makes collaborating between each person easier. It can be used directly during workgroup sessions as a graphic visualization tool, as well as a workflow management tool for action plans.
- Your documents will always be up-to-date, standardized, and easy to transmit: specifications, Design or Process FMEA studies, risk management reports, data to build the Usability Test Report, summary of actions, and updates to risk levels.
- Limit stress during unexpected audits as the coherence of your data is ensured!
To comply with the main regulatory requirements and standards for the French, European, North American, and international references, our software tool is qualified and valid for your risk analyses under ISO 14971.
We will also develop and provide a complete validation file for the RM 14971 tool as each new version of Medical Device Software is released.