For Regulatory Affairs Departments to
-
Manage medical device risks under ISO 14971:2019 and
-
Automatically generate risk management reports
RM 14971 is a Medical Device Suite database module.
An ISO 14971 program with integrated reference documents
This ISO 14971-based management risk software integrates contents from reputed references. It is based on the contents in the ISO 14971, ISO 62304, ISO 62366, and ISO 24971 standards, as well as the practical guide published by the members of the SNITEM in partnership with the CETIM.
The program helps you carry out your hazard, event, and physical injury risk analyses for equipment and environments following the CETIM guide and the current related standards.
Content is available in English and French.
The reference documents are contained in the libraries and can be modified and enhanced by your authorized users. You can add your specific references and use them for hazardous situations, actions to perform, and so on, as you progress.
We have designed the program structure and the risk analysis charts, in particular, in compliance with ISO 14971:2019. The column headings contain the references for the standard (with the chapter number related to the step in the analysis).
The tool uses these references to guide users through steps to ensure compliance with the applicable standards.
Different elements in the software program help verify if your evaluations comply with the requirements found in the standard. A dedicated report lists the essential chapters in the standard and shows you where to find possible shortcomings or incoherence in your risk analyses. For example, if you have unevaluated risk/benefits or unaccounted, and therefore reduced, combined risks, and so on.
Medical device risks under ISO 14971
When following ISO 14971, you must prepare a certain number of elements prior to carrying out your risk analyses. The program will guide you in the choices to make to define your organization, steps, and rules you would like to follow for the evaluation and for building your risk management plan.
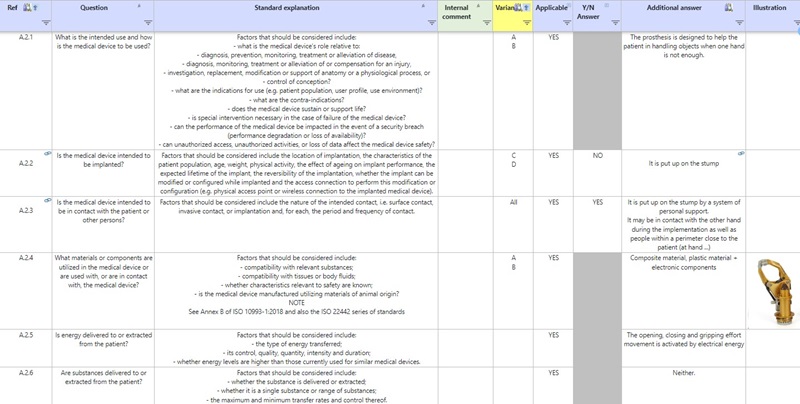
A chart helps you respond to a variety of questions to describe the intended use of the device in compliance with ISO 14971. You can perform this analysis for each device or for each family of devices by indicating any particularities, if they exist.
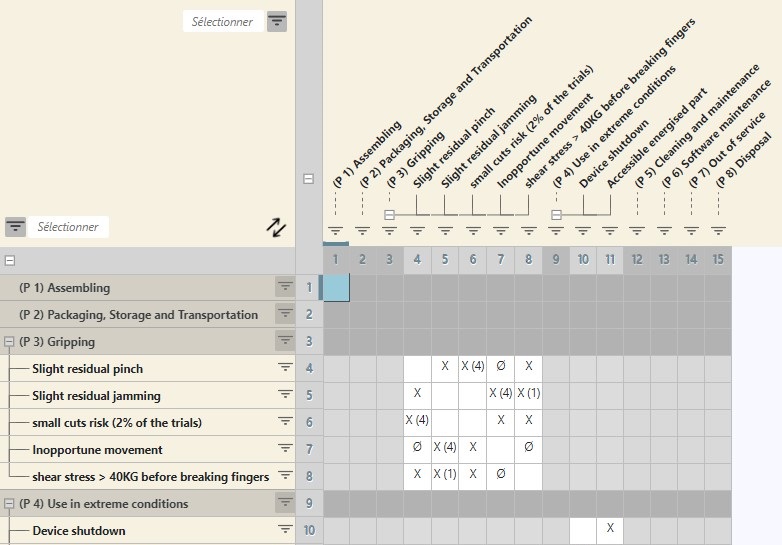
The RM 14971 tool elaborates risk analysis by following the life cycle of a medical device: design, manufacturing, shipping, storage, use, maintenance, end of life, etc.
It helps you identify, analyse, and control risks for each of these phases in the life cycle.
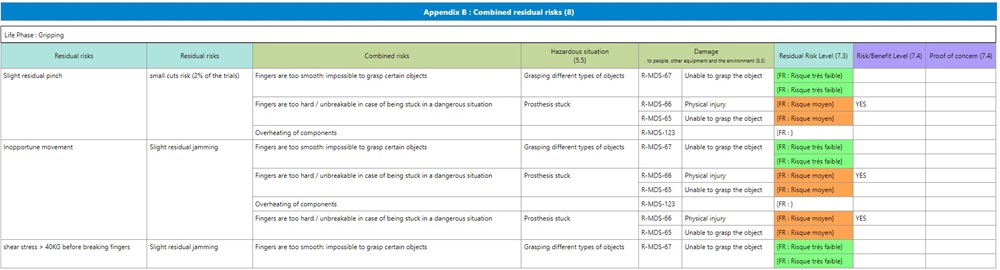
To make sure your analysis is complete, you can fully and easily consider the risks resulting from measures and the combined residual risks. You can use a matrix to identify pertinent residual risk combinations more readily.
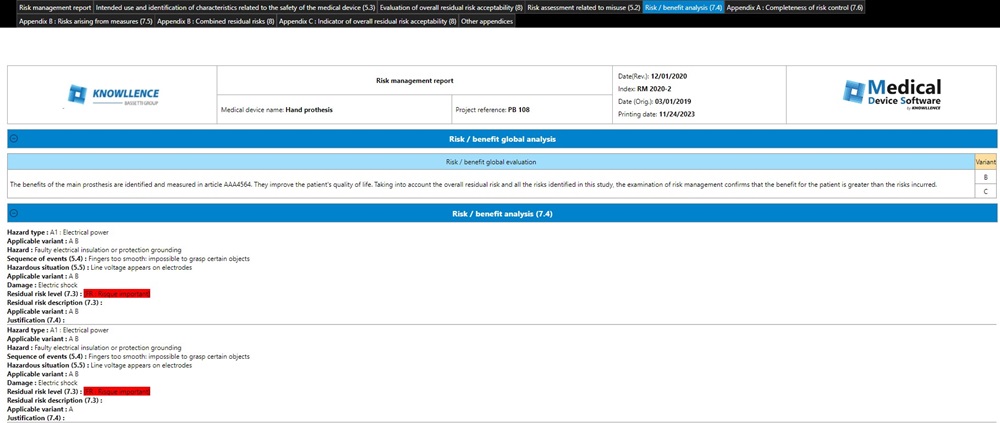
In compliance with the standard, the software program enables decision-making for each identified risk/benefit situation and for the acceptability of the overall risk in relation to this risk.
To top it all off, at the end of your risk analyses, you can add the evidence related to the overall risk/benefit analysis and the overall risk acceptability of the medical device.
The tool can trace the various risk management reviews you carry out, and you can also enter the approvals linked to these reviews.
If needed, a dedicated report records the different reviews you have performed for your project.
The program then automatically generates the risk management report with the structure following the standard’s requirements.
We also supply other documents to help you carry out your processes efficiently.
Centralization of risk management actions
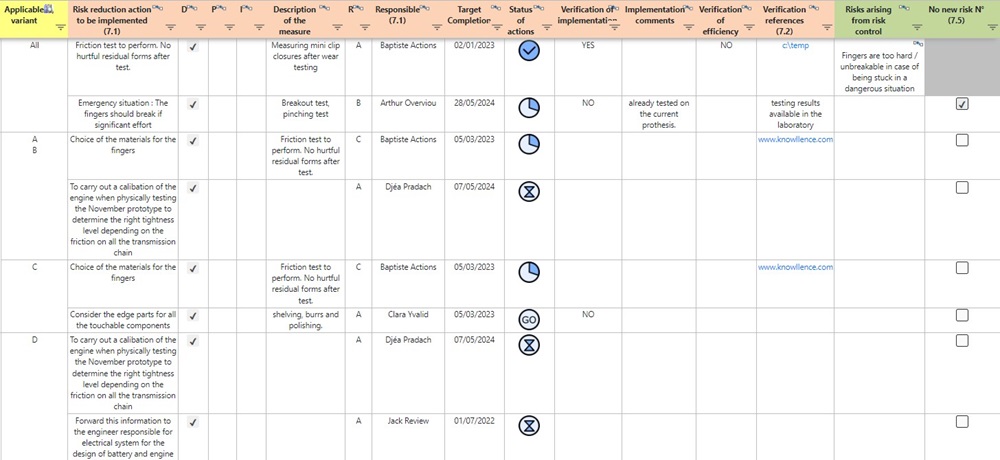
Medical Device Suite’s RM 14971 module helps centralize the monitoring of different action plans in a single database.
You can monitor the risk reduction measures you propose in compliance with the standard. The program considers the related verifications (implementation and efficacy), the proposed action category (design, protection, information), and the related efficacy level.
A collection of indicators and reports allows you to follow the progress of these actions and to control their implementation. Medical Device Suite also integrates global management of expenses for active projects.
Risk analyses integrated into your quality management system
With Medical Device Suite, your 14971 risk analyses will no longer be independent and separate from your other risk analyses (Process FMEAs, for example) or the other methods in your design/manufacturing cycle.
You can establish links between your various design steps to ensure coherence and efficient consideration of the impacts linked to any changes (especially post-market ones).
This makes it possible for you to link a hazardous situation identified during a 14971 risk analysis to a Process FMEA cause.
The tool comes with a validation file
To comply with the main regulatory requirements and standards for the French, European, North American, and international references, our software tool is qualified and valid for your risk analyses under ISO 14971.
We will also develop and provide a complete validation file for the RM 14971 tool as each new version of Medical Device Suite is released.